
- Support portal
- Evaluation Kits and partner products
u-blox Support
- Product documentation
Documentation
- Investor relations
Investor relations
William Saltzstein, President and Founder, Code Blue Consulting, and
Pelle Svensson, Market Development Manager, Product Center Short Range Radio, u-blox
The confluence of wireless technology and healthcare offers virtually unlimited potential for innovation. But the road to transforming an idea into a viable product and successfully bringing it to market is bumpy, with many barriers - technological, societal, regulatory, and security-related - that need to be overcome. In an interview, William Saltzstein and Pelle Svensson discuss the past, present, and future of eHealth.
Pelle Svensson – I would describe it as using existing electronics and communications technologies to provide health monitoring outside the hospital walls.

William Saltzstein – I've been in the eHealth business for almost 25 years. While it’s changed name, for instance, telehealth or remote patient monitoring, its goal has not changed. Basically, on the medical device side, it's a spectrum of solutions that starts in the hospital, expands all the way out of the four walls, and starts to merge today into personal health. It's not really a new concept, but I see it as being increasingly enabled by the technologies that we deliver, that is the wireless communications of various kinds that work together to provide solutions to do things like letting us stay in our homes more safely as we age.
P.S. – The European Commission talks about digital health, defining it as tools and services that use communication technologies to improve efficiency in the health sector and to cope with the increasing demand coming from population growth and more people aged 65-plus.
W.S. – We've seen that hospital infrastructure spending has been flat, in some cases even declining, and that we need the efficiency to deliver services outside the four walls of the hospitals. I think wireless solutions are the key enabling technologies that allow us to do that, safely and effectively.
P.S. – Yes, to be able to cope with the growing population and still be able to deliver good healthcare, eHealth is a must going forward. It's just a matter of how we can enable it quickly enough to make sure it gives us the benefits that we're aiming for.
P.S. – According to the World Bank, the population will reach ten billion in 2050 from today's 7.8 billion. The number of people above the age of 65 is expected to climb from five percent of the total population to 25 percent in three decades. Of course, this will result in a rising demand for health services and, specifically, higher costs forhealthcare.
W.S. – You've touched on one of the key points, and that's control of costs while being able to deliver these services. I see the pandemic as a driving force in pushing forward the solutions that we've needed, and in showing how they can be cost-effective. There's been a reluctance to invest in some of these technologies on a countrywide scale because the return on investment wasn't clear.
“I see the pandemic as a driving force in pushing forward the solutions that we've needed, and in showing how they can be cost-effective.”
What COVID has done is to put emphasis on being able to deliver these services more efficiently so that we can reach more people and, at the same time, protect our healthcare workers. It has pushed models forward to what we knew was necessary and accelerated the timeline. The good news here is that we have the technologies to be able to deliver these solutions and apply them to these new needs.
P.S. – I think technology has come to a point where microcontrollers, sensors, and communication are available and can deliver good quality. In a study published in The Lancet, I read that global spending in healthcare will reach close to $20 trillion in 2040. I think healthcare spending will become one of the biggest economic challenges going forward.
W.S. – The other thing is that COVID has shown the entire world that our communication networks are robust and can handle the increased data traffic. We had companies that use VPNs increase from hundreds of workers to tens of thousands of workers overnight, and our networks handled that increase quite effectively and securely, proving that our communication tools are up to the task. As challenging and awful as this pandemic has been, I think the real silver lining is that the whole world has seen that we are ready for these solutions, and this gives us confidence and the ability to move forward quickly.
P.S. – The coronavirus has affected every person's life, in school, at work, etc. We are working more from home, remotely. We see globally a lot of incentives among our customers, the device manufacturers, to make products that can assist in keeping physical distance to prevent spreading the virus. It's interesting to see that in a crisis like this, there are already people that are using their creativity to try to find solutions.
I read an article about telehealth in the US. One hospital in the US with about 3’000 monthly telehealth visits reached 60’000 telehealth visits because of the pandemic. So definitely, the technology is there. We just need to encourage the use cases to be more widely used. Of course, there are some companies and some new organizations that have to help, for instance, the insurance companies in the US and the governments and so on.
W.S. – We have seen the FCC (Federal Communications Commission) and FDA (Food and Drug Administration) working together to enable these solutions to get to market very quickly. We are seeing some very innovative ways that the government agencies in the US are working together to make things happen on accelerated timeframes, and companies that would have struggled prior to COVID are now seeing the funding they need to move their innovative solutions forward quickly. Human creativity is amazing. It takes a lot of hard work to put these solutions into the market. But the time is really good for new and innovative solutions.
P.S. – It's about sensors, microcontrollers, and connectivity. The data from the patients has to be delivered to the caregiver through the internet in one way or the other. I see at least two possible architectures, which I would call one hop or two hops to the internet. One hop means that you have cellular connectivity in a device that is monitoring the patient. Data goes directly through the cellular network to the internet and on to servers where caregivers can use the information. The two hop solution is more of a hybrid solution consisting of a short range technology from the sensor to a hub, which could be a home gateway or a smartphone app, and then from there to the internet, either through wired or wireless, in this case also cellular, technology.
“Human creativity is amazing. It takes a lot of hard work to put these solutions out. But the time is really good for new and innovative solutions. ”
W.S. – From the communications side, there is no one-size-fits-all solution. When new technologies come out, it's very tempting to say: "We're done now. We can focus on one single technology." I've been in communications for long enough to know that it still hasn't happened.
The challenge is, to use the medical device term, intended use: There is no single eHealth model. There can be different architectures, different technology combinations, all motivated by different user needs.
The technology needs depend on the intended use. Bluetooth is clearly the low power, low cost, first hop technology that is easy to use with a smartphone. But for something like an implanted device with no user interaction, it might be tempting to go straight to the cloud with cellular technology. If you are on the road transporting a patient, you might need to use 5G. And when you hit the four walls of the hospital, you have to switch to Wi-Fi, the preferred network there because of its installed base and roaming capabilities.
“The challenge is, to use the medical device term, intended use: There is no single eHealth model. There can be different architectures, different technology combinations, and different user needs.”
So you really have to evaluate each application, its needs, and pick the appropriate technologies. The good news is we have a lot to choose from. Everything from Bluetooth to Wi-Fi to 5G. We can add GPS technology to the mix to be able to locate people when they are ambulatory and to make sure that they aren't going near a store that sells peanuts if they have a peanut allergy, for example.
My main message here is that there is no single wireless technology today that can provide for all of these needs. And the benefit of a company like u-blox is that it offers the building blocks that you need to put together solutions that meet each application’s intended use.
P.S. – Exactly. Which technology is used depends on factors like: is it a battery-driven device that's going to be used frequently, or is it a stationary one that can be powered by the mains? Everything that uses two hops to the internet needs a gateway, which means that there are configuration aspects to consider, while a cellular modem implemented directly in the patient monitoring device can be configured in the factory. Just power up and it connects automatically.
W.S. – I was recently with a group of experts talking about the impact of 5G in healthcare, and the conclusion was that, while it is an excellent technology and another valuable tool, it is not, at least in the near term, going to be the one technology that solves everything. A challenge with 5G is the need to have the carrier involved in the conversation; something that medical device companies and hospitals aren't used to dealing with. How do you handle who pays for the service and who does the provisioning? And is the device and service available on all the networks? So there are still some questions to be answered about deploying 5G for medical device technology, particularly for continuous remote patient monitoring and more critical medical applications.
P.S. – There is constant evolution and development of new features for each technology, supporting new use cases. What we use in five to ten years will not be what we have today, but rather an evolution of the technologies we have today.
W.S. – We've seen that with the evolution of Bluetooth and Bluetooth low energy, then with the new modes, from Bluetooth LE Audio to Bluetooth Mesh.

P.S. – When implementing technology into medical devices, patient safety is important. In the US, the FDA is responsible for protecting public health and enabling the security of medical devices, while, in Europe, it is the task of the European Commission's Directorate-General for Health and Food Safety. Both publish information on making medical devices in general, and specifically wireless ones, providing guidance on how to provide patient safety, ranging from the choice of technology and where to use the device to how it can coexist with other potential technologies in the use case it's being developed for.
W.S. – The medical device world is pretty strictly defined between those applications that need to be regulated and those that don't. A blood pressure meter is a regulated device, while a weight scale is likely not, unless it is a part of a medical device system. Some devices cross the boundary like a pulse oximeter, which is a regulated device when used in hospitals, but not if you're going to use it for mountain climbing to make sure you are getting enough oxygen. There is a regulatory process that must be performed before you're able to market a medical device.
So the regulatory barriers to entry are very real. You not only have to submit it to the US, Canada, Mexico, EU, Japan, and China; each of these regions has the ability to impose regulatory restrictions and require approval. It's quite a task to figure out what it takes to make a medical product available worldwide. And, for wireless devices, these approvals require that you are granted radio approvals as well.
There's a very large change going on right now in Europe, with a transition to what's called MDR (Medical Device Reporting), which is a different set of regulations and a different process for being approved through notified bodies. And, of course, cybersecurity is a much more important part of the process than it used to be, for good reasons. When you have a wireless signal that can be sniffed and decoded, we need to make sure that we have the appropriate features in the communication channel to protect security and privacy.
Combined with that, we need to maintain supplier quality to ensure the quality levels required to keep devices running. Because medical devices are used in proximity to other electronic equipment, interference and coexistence of medical devices have also become key in “noisy” environments, such as in ambulances. And with more and more of these solutions, interoperability, having communications protocols that are able to inter-operate and exchange communications and data from one system to another, is going to be playing a bigger and bigger role.
P.S. – We as components suppliers have good examples over the last five years where we basically have all our already certified solutions and modules going back into the test facility to make sure that we are compliant with the latest key specifications both in Europe and the US.
P.S. – There’s a medical IT solution from almost 20 years ago, around the time that the term Internet of Things was being coined. We had customers in the medical area that were making devices that were used in ambulances to take sensor data from a patient, send it over a Bluetooth connection to a specially designed gateway, and then to a server on the internet. What that server did was re-package the information and deliver it to the hospital in the required data format. What is impressive is that these products are still in use, just with an updated form-factor-compatible wireless module.
W.S. – From my side, one really good example of where the future can go is the diabetes case. People with Type 1 diabetes need to monitor their blood sugar level and inject themselves with insulin to control it. The technology has evolved for decades, from hospital tests to finger sticks and the use of strips to be able to check the glucose levels frequently during the day and inject insulin.
Today, we have body-worn devices called continuous glucose monitors that can be worn for weeks at a time, continuously reporting on the level of blood sugar. The data can go to a smartphone app where users and their caregivers can make sure that they're perfectly managed. The other devices that have been developed are insulin pumps, which are external devices worn in a pouch that have a portal into the body to automatically deliver insulin. The two devices hadn't been able to work together until two to three years ago when several companies began marketing devices that use wireless technology to let the body-worn sensor deliver information to the pump and automatically keep the blood sugar levels within a very small window.
As you might expect, this benefits the patient greatly, not only because they don't have to do it manually, but also because, in the long term, studies have shown that good control of insulin and sugar levels produces benefits in terms of a longer and healthier life.
The other application that I'm really excited to see, because it's one that I actually didn't think would be a valid application for Bluetooth technology, is for implanted devices. Things like implantable cardioverter defibrillators, pacemakers, and neurological stimulation devices are implanted with a Bluetooth low energy interface connecting them to an external device such as a smartphone or external pump.
You can extrapolate about the future that we're going to have more devices helping us with chronic conditions going forward so that, as we age, we are healthier and less of a burden on the healthcare system. This is exciting. I think this is really a good example of where we're going that we would not have without the technologies we have developed in the past 20 years.
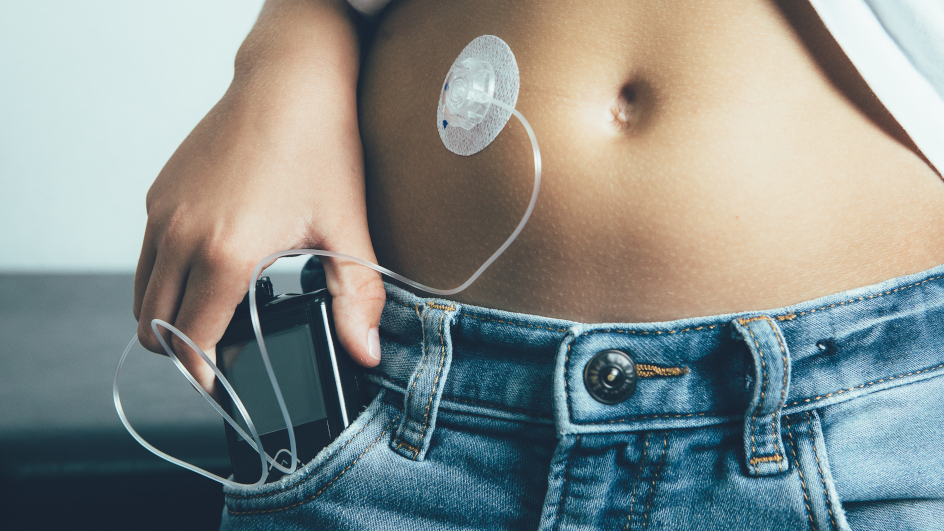
P.S. – If we take the example of devices designed to control diabetes, it brings up the question of hackers and security. At u-blox, we have a very strong security program in place delivering security as a service to have top-line security for devices that require it. First, it requires a high degree of protection from malware and data tampering. Patient confidentiality is essential too: only authorized medical staff should be able to access sensitive data.
At u-blox, we answer these imperatives with LPWA products that are secure by design, which means that they include a unique and immutable device identity tied with the root of trust (RoT), a source which forms the basis for a trusted set of advanced security functionality, which we offer as IoT Security-as-a-Service. This scalable solution is also specifically optimized for e-Health applications that use resource-constrained IoT devices.
W.S. – Cybersecurity has become more important for medical devices. As we become more dependent on interoperable technologies, they become somewhat easier to get into. So it's important that, at the level of a company such as u-blox, we take appropriate measures to make sure these devices are secure.
Another aspect that is occasionally lumped together with cybersecurity is privacy. The European Union has taken a great step forward with the GDPR to protect personal privacy. As some of these devices start to disclose our chronic conditions, we don't always want our neighbors to know about them. That's where privacy comes in. That's just a personal example, but there are other examples of where privacy is important, particularly when we start to have valuable patient information that is tied to the US social security number.

P.S. – According to a recent report by Berg Insight, the adoption rate of health systems is increasing. In the last year, we have seen strong growth in North America, followed by some countries in the rest of the Americas, some countries in Europe, and Japan. One reason why the US is leading the trend is that they have the highest patient treatment cost. It’s an incentive to use eHealth to still be able to provide the service for the patients. Other countries that recently joined the trend are China, Russia, and the Middle East.
If we look at the number of connected devices that are currently being used, we have 25 million in North America, with Europe representing 12 million devices, and the rest of the world representing five million.
But the report also looks at a few years ahead and says that the biggest increase will be coming from the rest of the world. North America and Europe will close to double in the next three to four years. In total, Berg Insight foresees doubling of the number of connected devices used in eHealth and mHealth use cases over the next three to four years.
W.S. – I think that sounds consistent with what I've been seeing. The US used to be responsible for almost 75 percent of healthcare devices. That's 20 years ago. Your numbers were very interesting to hear. According to them, we are at about half the world market and falling. And it's not necessarily because we're using less, but because the markets in the other parts of the world, particularly outside of the US and Europe, those in Asia and the Middle East, are growing.
There are many reasons for this. One is access to the devices and the technologies. Another is general economic trends. Perhaps the third piece is that, as countries develop, different healthcare issues pop up that weren't there before. Things like pollution and certain lifestyles tend to increase healthcare issues.
“AI and machine learning will play a big role going forward.”
The challenges I see going forward are the countries with large populations and low budgets. I think that we as an industry can work together to try and lower those costs and get into these markets that clearly have the need and be able to deliver those benefits.

P.S. – As we have seen, the use of eHealth has grown dramatically in parallel with the COVID-19 pandemic. And I think it's definitely here to stay, as the last couple of months have proven that the technology can deliver.
With more devices, more connectivity, and more data being collected and in need of being analyzed, there is a challenge. Will it be done by doctors? To some extent, yes. But I think artificial intelligence will come into play to do a pre-analysis of the data. Of course, the final word will always be on the doctor. And we will probably also see more implants being wirelessly connected not only to monitor patients from the outside, but also from the inside.
W.S. – I'm going to echo that. The data has to be turned into information. That information has to then be turned into assistance for decisions to make changes in healthcare or to say that someone is okay. We need to keep the physicians and other healthcare workers from getting overloaded. They need to quickly be able to understand what this information means, and how to deliver better care.
AI and machine learning will play a big role going forward. The algorithms to do closed-loop control, like the diabetes example that I gave, are going to be AI algorithms. They learn and adjust as you change your lifestyle and as you age and change.
“You should be able to have access to the expertise around the world. ”
P.S. – A trend I've seen here in Sweden in the last couple of years is doctors doing consultation using apps on smartphones. There are a couple of different companies providing this service. And it's being paid for by our medical system. We are 10 million people in Sweden. Over the last year or two, there were one million downloaded apps for one of the suppliers. So it's a very strong trend. Doctors and patients can talk to each other, use the camera in the phone for the doctor to examine a particular part of the body, and provide prescriptions for medication. This way, you don't have to go to the doctor to see the doctor. And now these companies are collaborating with pharmacies so that, if you really want to talk to someone in person, there will be nurses in pharmacies to complement the video conferencing.
W.S. – We're seeing some of that in the US as well. I think that's another important area. It's exciting to see countries like Sweden take the lead on that. The advantage Sweden has is that its healthcare system is well controlled, well funded, and well understood, whereas in the US, in my opinion, we're not any of those. I see that healthcare devices and the tools that companies like u-blox deliver can help people around the world be healthier, live better lives, collaborate more easily, wherever they are located. You should be able to have access to the expertise around the world.
We need communications to help move us forward. I'd like to see our devices and solutions extend to the struggling regions where people need the healthcare solutions that we enjoy. I'd really like to see healthcare without boundaries and without favorites. That’s my hope and vision for the future.